Authors: Mariana Sperotto*, Melissa Heinen*, Karoline Von Wurmb*, Bibiana Braga*, Rubens Bisatto
*Dorf Ketal Brasil
Introduction
Hydrogen sulfide (H₂S) is produced in oil reservoirs through bacterial and thermochemical processes. Even at low concentrations, H₂S is toxic to humans and can cause significant corrosion of pipelines, flowlines, and equipment. Therefore, its removal during oil extraction and production is crucial. Several methods exist to remove H₂S from oil production lines, with the most common being the injection of chemical H₂S scavengers. These scavengers react with H₂S to form less toxic and less corrosive byproducts (Obakore et al., 2017).
There are two main types of H₂S scavengers: triazines and glyoxal. More recently, hemiacetal has emerged as an alternative, developed to yield a nitrogen-free product with a more neutral pH. A hemiacetal is formed through the reaction of an alcohol with an aldehyde. Among hemiacetals, the bis-hemiacetal, created by reacting ethylene glycol with paraformaldehyde or formaldehyde solution, is the most widely used. This type of molecule is effective as an H₂S scavenger because it releases formaldehyde. Additionally, it is nitrogen-free and has low corrosivity (Wylde et al., 2020).
Despite its widespread use, several factors during the synthesis of bis-hemiacetal-based H₂S scavengers influence the level of free formaldehyde. These factors include the type and amount of catalyst used, the molar ratio of ethylene glycol to formaldehyde, the water content, the temperature, and the reaction time.
Formaldehyde, the simplest aldehyde, is among the most widely produced chemicals globally. However, it is toxic and classified as carcinogenic by the International Agency for Research on Cancer (IARC). Due to its high volatility, formaldehyde can easily enter the airways, and numerous studies have linked it to nasopharyngeal and lung cancer. Consequently, workplace exposure to formaldehyde must be controlled, with the World Health Organization recommending a maximum concentration of 0.08 ppm over half an hour. In Brazil, according to Regulatory Standard (NR) No. 15, the maximum exposure limit is set at 1.6 ppm for a 48-hour work week. To address this issue, the European Union has implemented the REACH regulation, which establishes a limit of 0.1% free formaldehyde in chemical products of this category (Viegas, 2009; Romão, 2018).
In this study, various synthesis routes were explored to produce a bis-hemiacetal with low free formaldehyde content. The resulting samples were evaluated for free formaldehyde content using NMR spectroscopy, assessed for H₂S scavenging capacity, and tested for stability under subsea conditions. Additionally, conventional H₂S scavengers were compared with the newly developed ones.
Methodology
Initially, various synthetic routes were evaluated to produce a bis-hemiacetal product with minimal free formaldehyde. NMR was used to analyze the resulting products and quantify free formaldehyde levels. Following this, the selected samples underwent efficiency evaluations using the autoclave method with continuous injection of CO₂/H₂S gas. To validate the synthesized scavenger for subsea applications, it was subjected to a specific protocol designed for pre-salt conditions.
Assessment of H₂S Removal Efficiency Using Parr H₂S in an Autoclave
The methodology involves subjecting the fluid to a constant flow rate of 600 mL/min using a gas mixture containing CO₂ contaminated with H₂S at 32,000 ppm, under predetermined temperature and pressure conditions. The concentration of H₂S in the gas phase is monitored by gas chromatography every minute. Once the system reaches steady state, an aliquot of the H₂S scavenger is added via an injection loop. This scavenger reacts with and consumes the H₂S in the fluid, and the H₂S concentration is monitored for at least another 60 minutes. Subsequently, the effluent gas stream will show a depletion of H₂S while the system re-establishes saturation.
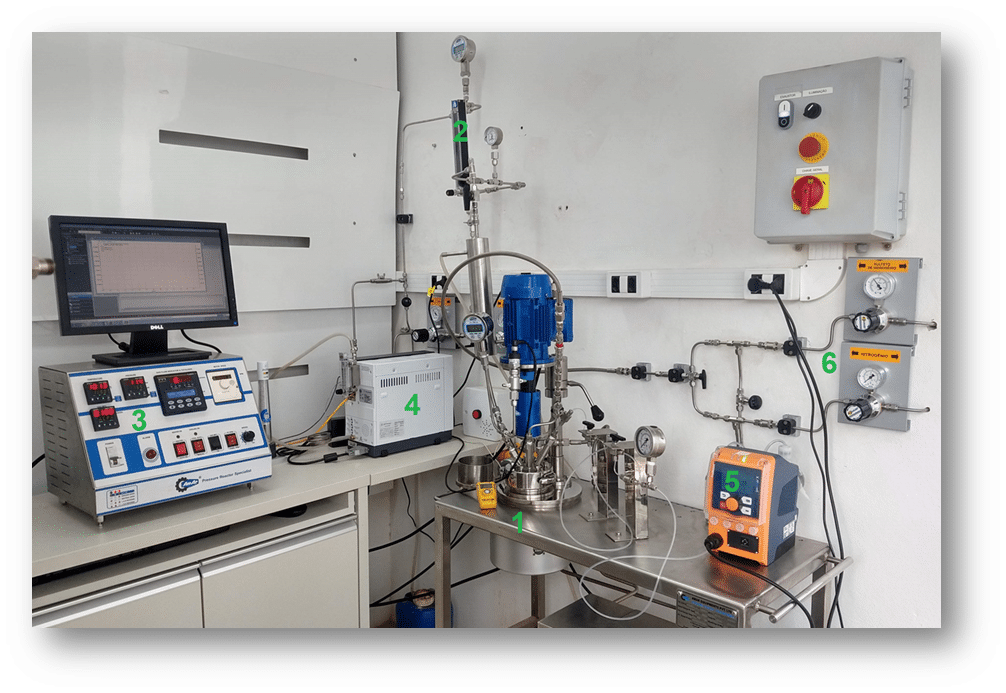
The capacity for H₂S removal is measured as the ratio of the volume of the scavenger to the mass of H₂S removed, expressed in liters of product per kilogram of H₂S removed (L product/kg H₂S). A lower ratio indicates a more efficient product. The mass of H₂S that is scavenged is determined by integrating the H₂S concentration curve over time.
This testing methodology is semi-quantitative and is used to rank various H₂S scavenger chemicals when tested side by side under identical conditions as a comparative assessment.
For this study, the pre-salt Brazilian conditions described in Table 1 were utilized for the H₂S scavenging tests, with the product dosage fixed at 1,000 ppm.
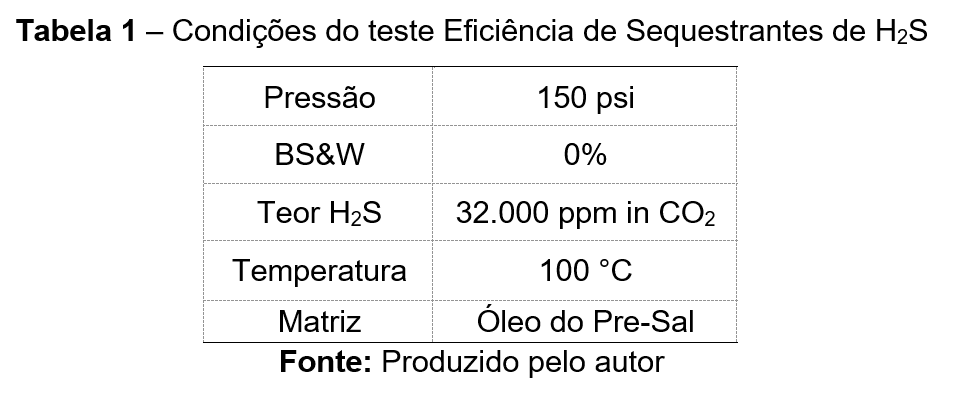
The table describes the test conditions for evaluating H₂S scavenger efficiency.
Product qualification protocol for subsea injection via umbilical
Product efficiency in the oil production process improves with longer residence times. Therefore, H₂S scavengers are typically injected subsea through umbilicals. However, the costs of maintenance interventions for subsea injection systems are very high, necessitating a set of specifications and best practices to ensure their safety and functionality. Consequently, products intended for subsea injection must undergo a pre-approval process in accordance with a testing protocol.
The following tests are required as part of this product qualification protocol for subsea injection:
- Static thermal stability at both low and high temperatures for 30 days.
- Product centrifugation at low and high temperatures for 7 days.
- Dynamic stability over 14 days in flow loops with in-line filters, under high pressure, and circulating through varying temperatures.
- Solvent loss at high temperature for 8 days.
- Compatibility with solvents at low and high temperatures for 24 hours.
- Dynamic viscosity measurements at low temperature under both low and high pressure.
- Assessment of product corrosivity towards materials commonly found in the umbilical for 30 days at high temperature.
- Compatibility with non-metallic materials in the umbilical for 90 days at high temperature.
- Verification of product composition to ensure the minimum required content of hydrate inhibitors.
Evaluation of H₂S removal efficiency via autoclave versus free formaldehyde content
After carrying out various bis-hemiacetal syntheses and analyzing the free formaldehyde content, two samples were selected for H₂S scavenging tests: one with low free formaldehyde content (maximum 0.1%) and another with high free formaldehyde content. In addition, three samples of conventional market hemiacetal-based H₂S scavengers were evaluated for efficiency and free formaldehyde content in the liquid phase.
Table 2 presents the free formaldehyde content determined by NMR spectroscopy, along with the H₂S removal efficiency of the selected samples.
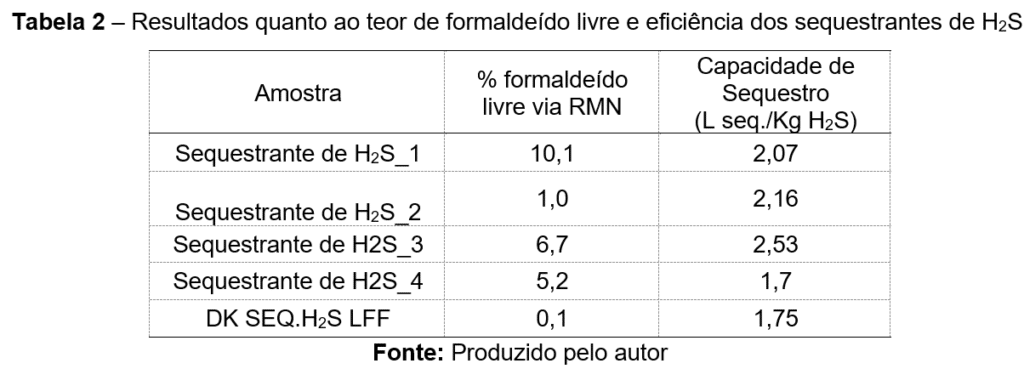
The table compares five H₂S scavenger samples based on two key parameters: free formaldehyde content (measured by NMR) and H₂S scavenging capacity (expressed as liters of scavenged gas per kilogram of H₂S scavenger).
Figure 1 shows plots of the H₂S scavenging tests, illustrating the relationship between H₂S concentration and time. Exceptional removal capacity results were achieved with a product that has low free formaldehyde content (DK H2S SVC LFF).
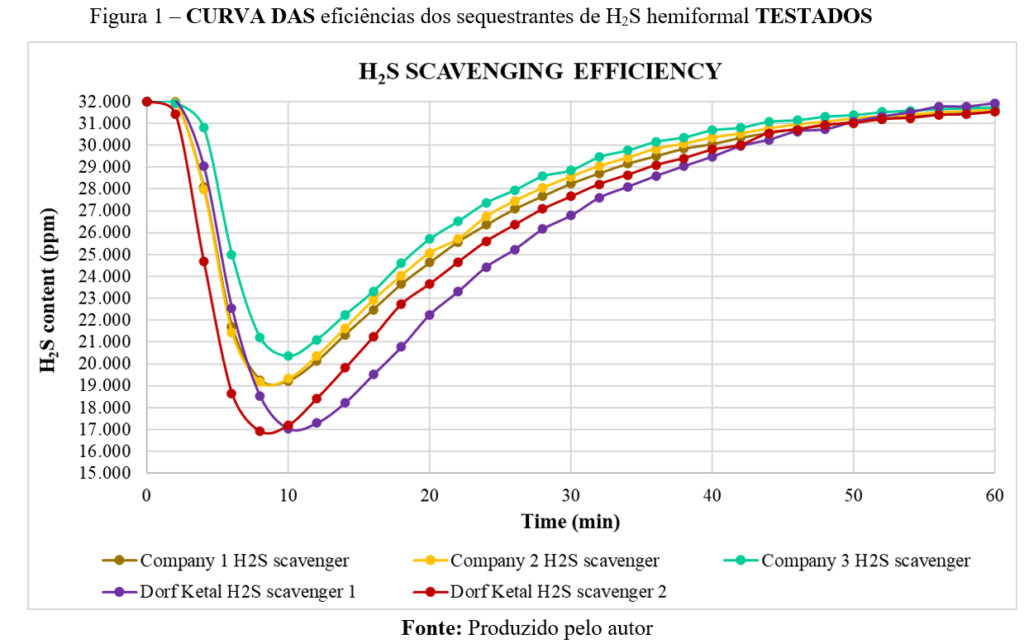
The figure illustrates the H₂S scavenging efficiency curves for different products over time. The vertical axis shows H₂S concentration (ppm), while the horizontal axis represents time in minutes.
Product Qualification for Subsea Injection via Umbilical
The H₂S scavenger, with a low free formaldehyde content, was assessed in accordance with a qualification protocol for subsea injection. Only the main results are presented here. Table 3 displays images illustrating the static thermal stability at 80 °C and 4 °C over 30 days. Table 4 presents images from product centrifugation conducted at both 4 °C and 80 °C for 7 days, under an acceleration of 1000 g.
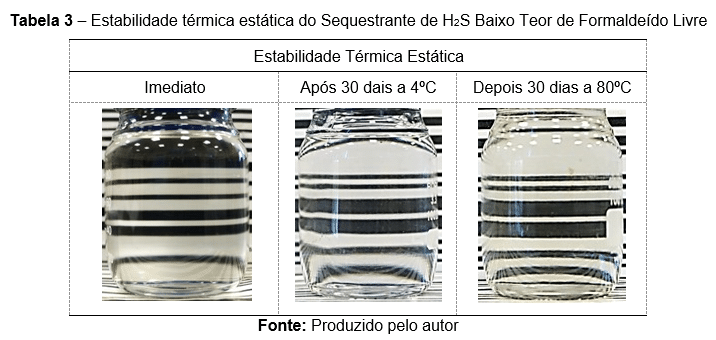
The table illustrates the static thermal stability of an H₂S scavenger with low free formaldehyde content under different conditions.
Even under these extreme conditions, the product retained its appearance, remaining homogeneous with no precipitates or phase separation.
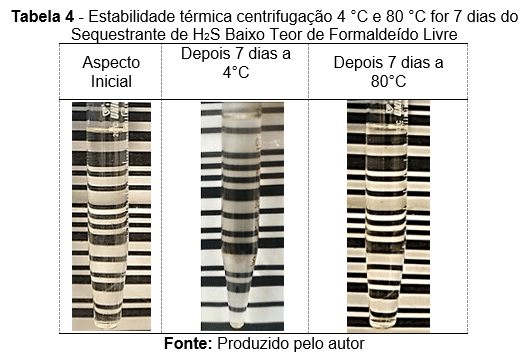
The table presents the thermal stability under centrifugation of an H₂S scavenger with low free formaldehyde content after exposure to different conditions.
To assess solvent loss from the product and the potential formation of solids during this process, an evaporation test was conducted in a U-tube. After heating the sample for 8 days at 80 °C, a slight volume loss was observed, as detailed in Table 5. Importantly, this loss did not alter the product’s characteristics; no sediments or precipitates formed, nor was there any increase in viscosity.
The table shows the solvent-loss test results for an H₂S scavenger with a low free formaldehyde content.
To ensure the safety and integrity of application line equipment, it is necessary to assess the corrosiveness of chemical products. Tests with metallic materials were conducted according to ASTM G31, and the results are provided in Table 6.
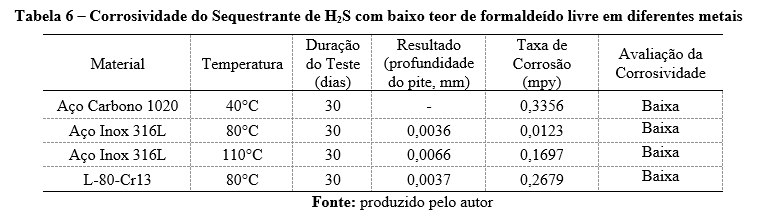
The table summarizes the corrosivity of an H₂S scavenger with low free formaldehyde content when tested on different metals under varying temperatures for 30 days
When evaluating subsea installations, it’s important to assess not only metallic materials but also non-metallic ones. In this study, we examined Nylon Besno P40 TLO, PVDF Kynar Flex 2750, and TFE/P materials by immersing them in the product for 90 days, in accordance with ISO 23936. After the immersion period, we analyzed parameters such as mass, volume, appearance, and mechanical properties, comparing them with those of non-immersed samples. We found that the H₂S scavenger was compatible with all tested materials.
We also evaluated the product’s viscosity under four conditions: 4 °C and 40 °C at atmospheric pressure, and 4 °C at 400 bar. In all conditions, the product’s viscosity remained below the 100 cP limit. Furthermore, it is noteworthy that the product contains the minimum required amount of hydrate inhibitors and is fully compatible with ethanol and monoethylene glycol solvents, even at temperatures ranging from 4 °C to 80 °C when exposed for 24 hours.
Final Considerations
This work demonstrates the following:
Several H₂S scavengers on the market contain high levels of free formaldehyde, which poses a health risk to operators who handle these products daily in the oil and gas industry.
A bis-hemiacetal H₂S scavenger can be produced with a very low free formaldehyde content of just 0.1%.
A product is available that exhibits excellent scavenging performance, achieving 1.75 L of scavenger per kg of H₂S. This product has also been qualified for subsea injection under the extreme conditions outlined in the protocol test results.
Increasing the level of free formaldehyde in hemiacetal H₂S scavengers does not improve product removal efficiency.
Acknowledgments
The authors would like to express their gratitude to DORF KETAL Ltda for the support provided during the development of this work and for granting permission to publish this article.
This article was presented at the Rio Oil & Gas Expo and Conference in 2022. | ISSN 2525-7579